Designing for care delivery
Pioneering a service model to deliver precision medicine from lab to patients
Background
Healthcare transformation is accelerating. Advances in precision medicine, particularly radioligand, cell, and gene therapies, are rapidly reshaping what "treatment" means to both individuals and healthcare industries. Instead of manufacturing drugs at scale, pharmaceutical companies are expected to deliver highly orchestrated, end-to-end treatment experiences for individual patients.
However, most pharma companies are not ready for the challenge with their infrastructure today. This wasn't a marketing problem or a logistics puzzle. It was a service design challenge disguised as a product launch. And the clock was ticking.
"Gilead's CAR-T therapy, Yescarta, experienced significant adoption delays caused by a lack of coordinated infrastructure and service readiness—despite its proven clinical success."
Role & Context
Engagement length: 14 months
Role & Responsibility: Leading Service Design & Strategic Localisation
Team: Global CX strategy team & cross-functional design, research, product, tech, and medical SMEs
Client: Leading pharmaceutical company
My Mandate
- Define the global RLT service model—vision, experience principles, end-to-end blueprint
- Align the organisation around a shared understanding and readiness for change
- Enable global → local scaling across 5+ priority markets
- Create the operational ecosystem, roadmaps, and toolkits required for sustained delivery
- Building internal capability so teams could continue scaling the service independently
Challenge
A leading pharmaceutical company asked us to launch their breakthrough radioligand therapy (RLT). The brief seemed straightforward "We need an ordering platform for the successful delivery of this therapy"
But a rapid discovery phase revealed a deeper challenge. I'm not tasked with designing an ordering tool, but designing for the real need: an end-to-end service ecosystem required to deliver it successfully and sustainably across global markets.
Here's what made it truly complex: the organisation didn't think this way. Teams were siloed by function. "Customer experience" meant sales support, not patient outcomes. No one had ever designed a service where the entire treatment journey was the product.
My job was to make them see it, and then build it.
Execution
Service design orchestrated the program across multiple layers: global vision, product roadmap, market localisation, and operational readiness.
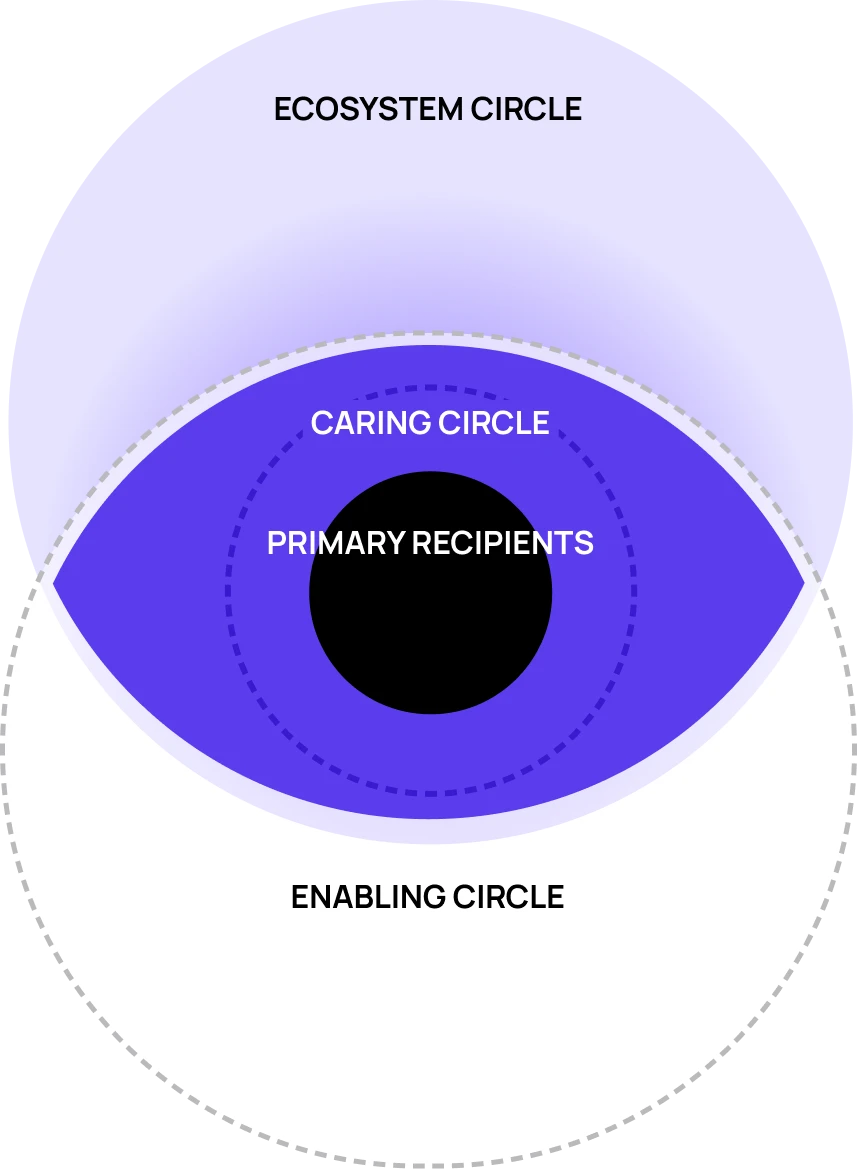
Make the invisible visible
Bring clarity and alignment into the complex treatment landscape
Everyone agreed the therapy was critical. No one agreed on what "successful delivery" meant. Through multi-market research, cross-team workshops, and strategic synthesis and journey mapping, we created the first shared understanding of how RLT must operate as a service.
Medical cared about clinical protocols. Commercial wanted market metrics. Supply chain obsessed over timelines. IT thought this was a portal project. As one Health Care System team member said "I had no idea supply chain was dealing with this…"
The clarity convinced leadership and teams across medical, CX, supply chain, IT, and commercial to finally saw the same system and rally around one unified vision.
The alignment didn't happen overnight, It took several rounds of working sessions to build a strategy and vision that honored each domain's expertise, gave leadership the full picture, and reflected what HCPs and patients actually needed.
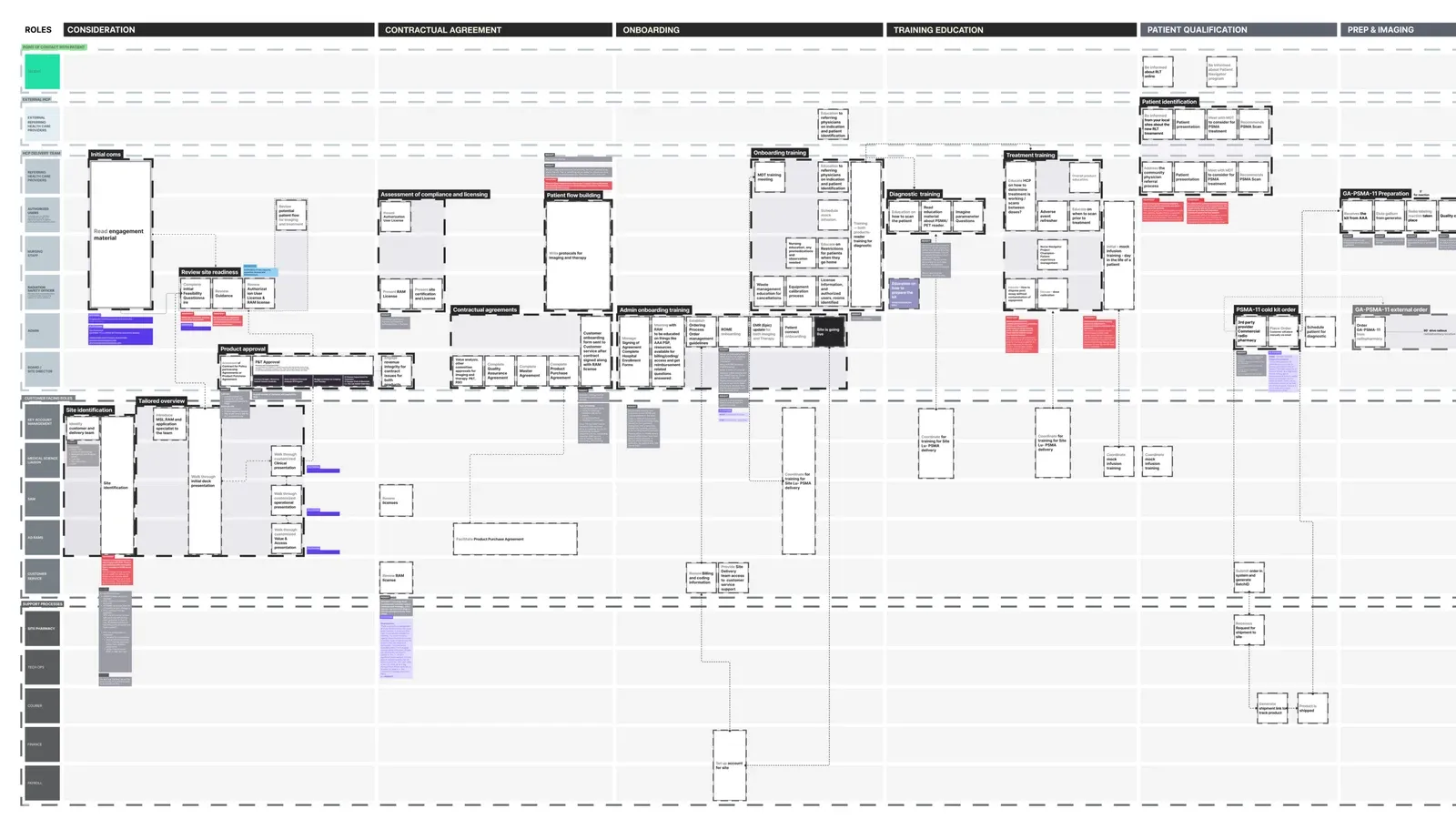

From vision to life
Turning a beautiful blueprint into an actionable product roadmap
A compelling vision is far from enough, it must be executable. I partnered with product designers to translate service moments into concrete product requirements, outlining:
- Features backlog
- Global vs. local archetypes
- RACI, Sequencing and dependencies
- Experience flows and service interactions
This became a prioritised product roadmap with a clear view of not just what we'd build, but also why it mattered to the service.

Design for reality
Localising the service for market nuances
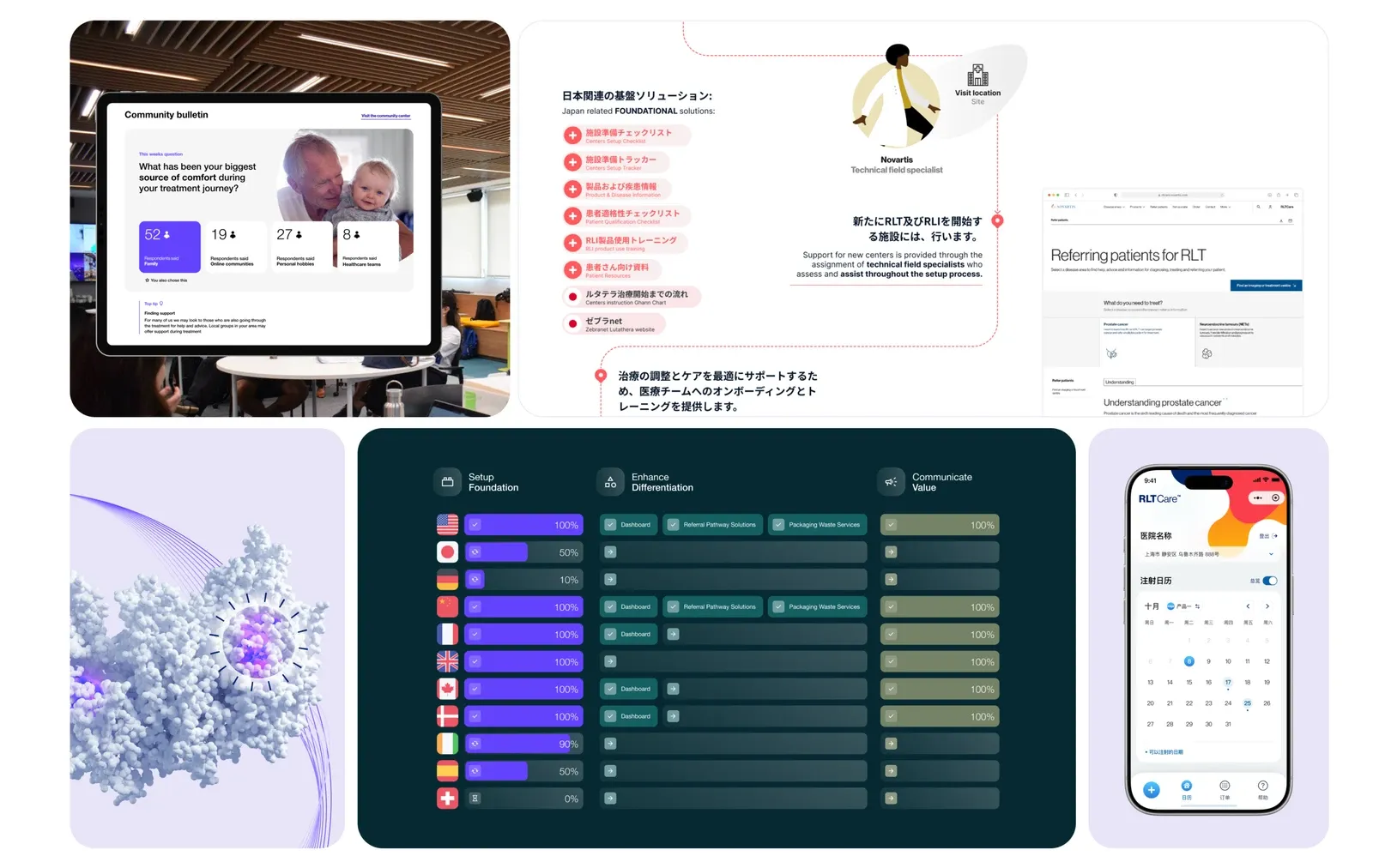
RLT delivery varies dramatically by geography. Regulations, clinical pathways, patient expectations, and infrastructure differ across markets. For instance, the referral network that works in Brazil simply won't work in Germany.
I developed a structured localisation methodology: a flexible framework with core principles and adaptable components. I ran workshops with top five priority markets, tested and evolved the approach. This accelerated readiness, reduced ambiguity, and ensured the service was culturally, clinically, and operationally relevant in each market.
Empower within
Operationalising and scaling the service
A service of this scale requires more than design. It requires the capability to sustain it.
Working with the central RLT teams, I built the operational backbone for long-term success. It includes prioritisation and governance framework, a repeatable implementation toolkit and a comprehensive package to onboard new markets rapidly.
This transformed the organisation's readiness from theoretical to operational, establishing the people, processes, and tools required to scale RLT consistently across the globe.

Impact
Delivery
- Oneunified end-to-end service model as the organisational standard for advanced therapies
- 2cross-functional strategy and alignment workshops
- 6+deep-dive country engagements
- 42+designed and validated service touchpoints & features
- 85+stakeholder and clinician interviews
Value
- 25%increase in reach to eligible patients in priority markets
- 8%faster expansion of treatment centre and referral network capacity
- 21%reduction in time-to-treatment across key geographies
- 83%+satisfaction from HCPs on clarity, reliability, readiness
- 4+global life sciences conferences featured
Learnings & Reflections
Balancing bottom-up insights with top-down sponsorship
Markets know the reality. Leadership controls the levers. We often sat in the middle, getting both sides to move in the same direction.
Change feels uncomfortable
We asked compliance-driven pharma to try creative ways of thinking, while requiring design team to learn clinical terminologies. Everyone had to stretch. Trust made that stretch possible.
It could be done differently now
With today's AI and agentic capabilities, many of the workflows we built could now be further accelerated or reimagined.